The financial benefits of using lignin recovered from wood as a biofuel at a kraft mill are obvious. However, lignin is being seen as a much more valuable element in the production of advanced bioproducts and biochemicals.

The most quantifiable benefits to a kraft mill for installing a Lignin Recovery system are the ability to debottleneck a recovery boiler to increase pulp production and to have a sustainable biofuel to replace fossil fuels in the lime kiln. The enhanced revenue from increasing pulp production and the cost savings from substituting purchased fossil fuels, in addition to the environmental impact of reducing greenhouse gas emissions, can be readily calculated to determine the payback.

of Lignin Recovery alternative concepts in
Shown here is the portable pilot plant
that can be easily transported to mills.
Today, with the increased knowledge and emphasis on biorefineries and bioproducts, potentially there is a new revenue stream. Lignin is now utilized as a sustainable, renewable raw material for advanced bioproducts such as composites or as a substitute for phenolic and aromatic compounds (Figure 1).
The challenge in making the Lignin Recovery technology cost-competitive has been to reduce the cost associated with removing excess sulfur that is introduced in the chemical recovery cycle with the addition of sulfuric acid used in the process. This cost is primarily the NaOH needed to make up for sodium losses that occur when sulfur is discharged with recovery boiler fly ash, which consists mainly of Na2SO4 and Na2CO3.
Beginning in 2010, ANDRITZ worked on the development of alternative process concepts and investigated them at pilot scale in several pulp mills (Figure 2). Today, it offers tailor-made solutions for Lignin Recovery, customized for each application out of proven components (Figure 3).
The system can be configured as one stage without washing, two stages with acid washing, or two stages with acid washing and drying. The exact configuration is based on the system infrastructure of the mill and the end use of the lignin to be produced.
The process is designed to remove lignin from black liquor at 35-45% dry solids having a typical pH of 12-13. After filtration and washing, the dry solids content of lignin is typically 60-62% before the drying stage, and 95% after. In its most complete form, the Lignin Recovery process consists of these sub-processes:
1). PRECIPITATION: The pH of black liquor from the evaporation plant is decreased with carbon dioxide (CO2) in the acidification reactor to precipitate the lignin from the black liquor. The resulting slurry is fed to a Membrane Filter Press that efficiently separates the precipitated lignin and lignin-lean black liquor.
2). ACID WASH: Impurities (mostly sodium) are leached from the compressed precipitated lignin in a dilution wash with sulfuric acid. Displacement washing is used to remove the sodium sulfate formed during the acid wash. Counter-current washing minimizes the consumption of fresh water as well as the amount of filtrate recycled to the evaporation plant. The displacement wash is carried out with pressurized hot water from the black liquor cooler and without additional chemicals to ensure that no new sulfate molecules are formed. A second Membrane Filter Press is utilized to dewater the slurry.
3). DRYING: The moist lignin cake is fed through a disintegrator for proper size reduction and to increase the surface area before the dryer. The heat source for drying is mainly the flue gas from a lime kiln or recovery boiler, which also acts as an inert drying media.
While producing high-quality lignin, the amount of sulfur in the chemical recovery cycle is increased considerably due to the large amount of sulfuric acid consumed in the acid washing step. This means that the excess sulfur must be dumped in order not to increase the sulfidity of white liquor. When sulfur is dumped in the form of recovery boiler fly ash, the amount of NaOH required a make-up chemical is high (up to 20-30% of the total cost of produced lignin). For this reason, it makes sense to combine the Lignin Recovery system with a Wet gas Sulfuric Acid (WSA) process (Figure 3).
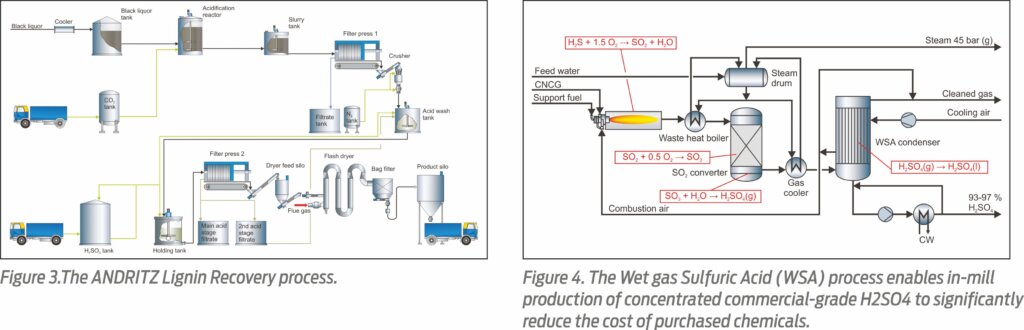
In the WSA process, commercial-grade sulfuric acid is produced on-site from CNCGs by catalytic conversion and condensation. There are over 130 references for the WSA technology operating worldwide on a variety of sulfurous gas streams, but none in the pulping industry. Up to 99.9% of the sulfur in CNCGs can be converted to concentrated sulfuric acid.
Depending on the downstream use of the lignin, ANDRITZ tailors the processes to achieve a mill’s exact goals. As with many ANDRITZ solutions, the company can deliver a complete plant – with the process design; main equipment including presses, crushers, dryer, bag filter, and lime kiln burner; as well as automation, and erection – from one source.
(Source: ANDRITZ AG. The Article was first published in SPECTRUM: Issue 35/1-2017: Tech Talk. SEPECTRUM magazine is published by ANDRITZ PULP & PAPER twice a year to discuss the main issues facing the pulp, paper and power industry.)



